

you need to be able to determine bonding in molecules to solve problems This specific heat calculator is a tool that determines the heat capacity of a heated or a cooled sample. you need a table of bond energy (enthalpy) values to solve problems breaking bonds requires energy (endothermic) and making bonds releases energy (exothermic) : if the ΔH value is negative, the overall reaction is exothermic if positive, the overall reaction is endothermic Check out the vapor Pressure of water calculator available online only at BYJUS to solve the problems easily. Once you know the total energy required, calculating the watts to heat the water is simple. ΔH=∑(reactant bond energies)−∑(product bond energies) In this case, the heat released (or the enthalpy of reaction ΔH) can be calculated by: Temperature (deg C) Pressure (atm) Submit. Many chemistry teachers start with bond energies. I am guessing you are looking at eitherġ) measuring by experiment using calorimetryģ) calculating using standard heats of formation More information is required as there are a few different ways to determine the enthalpy change of a chemical reaction. Hess's Law, building reaction mechanisms using enthalpies of reaction from thermodynamic tables. Is less accurate, though, because bond enthalpies are listed asĪverage bond enthalpies, and they depend on the particular You can calculate bonds formed - bonds broken. You probably won't learn this until sometime in college, but I thought I'd leave this here. (N/2)RT is the equipartition theorem, and the idea that (N/2)R = Cp assumes Cp varies negligibly with temperature.ĮX: Water vapor would have Cp = (6/2)R = 3R. The heat capacity may be experimentally determined (a fit line equation), or you can assume ideality for some gases and say that (N/2)R is equal to Cp, where R is 8.314472 J/mol*K and N is the degrees of freedom 3 translational for monatomic gases, 3 translational and 2 rotational for diatomic linear gases, and 3 translational and 3 rotational for multiatomic nonlinear gases (vibrational and electronic contributions are negligible in many cases). Using the heat capacity of a compound at a constant pressure (Cp) and integrating from one temperature to another, times dT.
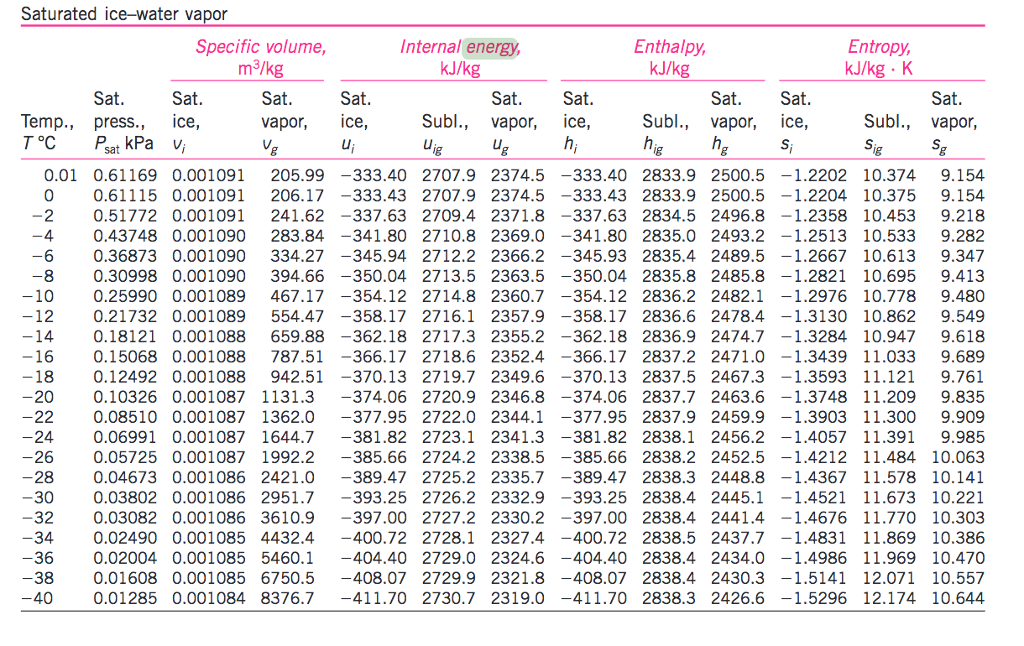
You are using the way of looking up enthalpy values in thermodynamic tables, like enthalpy of formation.ġ. However, depending on the unit, you may be forced to either multiply H by moles (when unit is Kj/mol) or leave it as it is (when unit is Kj).Īt first, you count H for finals, then for "ingredients" (by addition) and substract results. This calculator calculates thermodynamic properties for water as function of pressure and temperature. This process takes a tremendous amount of energy, and that energy accounts for the large amount of energy it takes to boil water to make steam in electrical generating plants of all kinds (including nuclear), and for the efficient means humans have of cooling our bodies: perspiration.Values of H (enthalpy) for particular reactants or reactions will always be given in the exercise. Notice that the largest contribution to this energy, by far, is in evaporating the water - changing it from liquid to gas. Here we use the heat of vaporization of water: Step 2: Convert the liquid water to steam at 100˚C. The saturation vapour pressure is the pressure at which water vapour is in thermodynamic equilibrium with its condensed state. Below we'll do an example of a heat calculation as the temperature of a substance rises through a phase change. The Wikipedia page of a compound is usually a good place to find them. Compared to most other substances, it takes a large amount of heat to melt water ice and to boil or evaporate water.Įnthalpies of fusion and vaporization are tabulated and can be looked up. The relatively large attractive intermolecular forces between water molecules gives water very high heats of fusion and vaporization. Water has no more phase transitions after this. Finally, gaseous water above 100˚C absorbs heat, increasing its temperature at a constant rate. This is the latent heat of vaporization, ΔH v, the energy it takes for water to have no more cohesive force.Į. Water at 100˚C absorbs a great deal of heat energy at 100˚C as it undergoes a phase transition from liquid to gas.
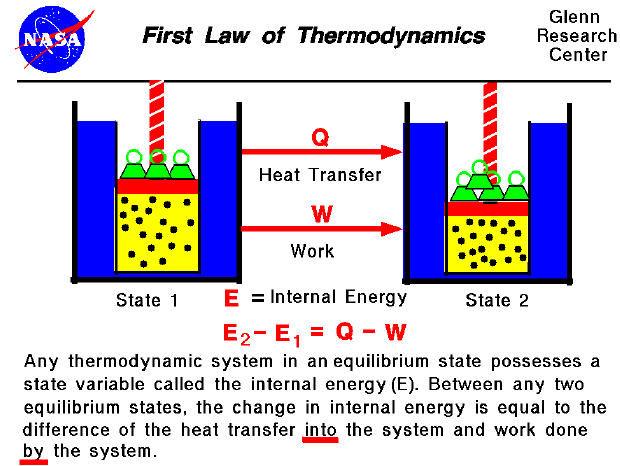
Heat is added to liquid water above 0˚C, and its temperature rises at a constant rate until the boiling point at 100˚C.ĭ. During the addition of the latent heat of fusion ( ΔH f), no temperature rise is observed, but hydrogen bonds holding the ice together break.Ĭ.


 0 kommentar(er)
0 kommentar(er)
